- November 27th, 2020
- Category: Finance
November 2020: OptimaT appears in VOKA magazine with new ISO13485: 2016 certificate
OptimaT achieves strict ISO standard for medical devices. Read the article from the VOKA magazine here:
November 2020: OptimaT appears in VOKA magazine with new ISO13485: 2016 certificate
OptimaT achieves strict ISO 13485:2016 standard for the assembly of medical devices.
Read the English translation of the article here:
"With ISO 13485: 2016, we once again demonstrate our great attention to quality"
OptimaT achieves strict ISO standard for medical devices
Supply company OptimaT from Lichtervelde can turn out to be a strong achievement: it recently achieved the ISO 13485 standard. That is an international quality standard for the production of medical devices. “This not only proves that we are driven by quality. Certification can also open new doors for us, ”says general manager Hein Moerman.
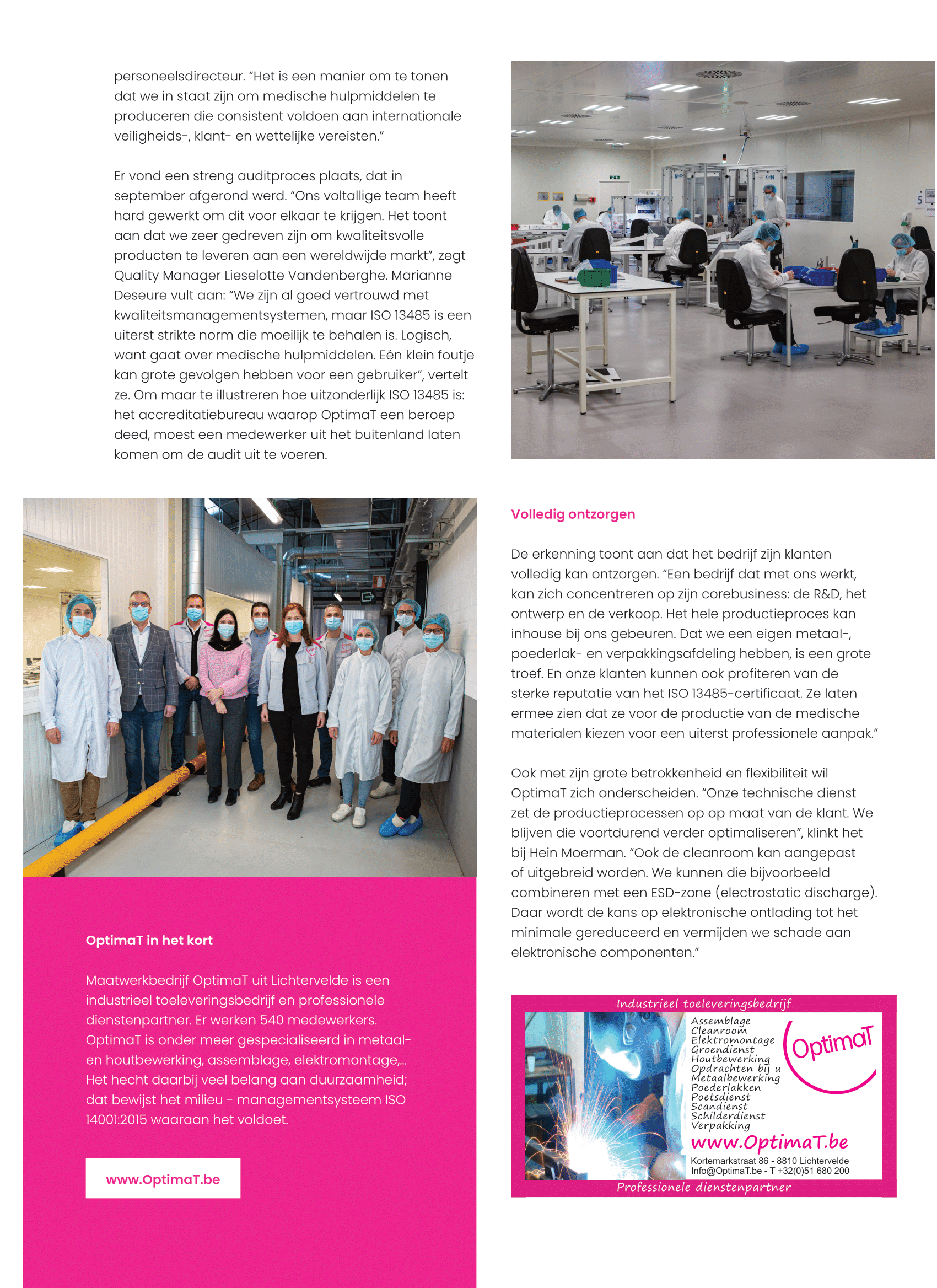
A few years ago, OptimaT already opened a SAR, a dust-free space. “It turned out to be very useful, so we wanted to go one step further,” says Hein Moerman. “Since last year we also have a cleanroom available, where pollution in the air is limited within certain limits. All environmental factors are strictly controlled. That makes it a safe environment for the assembly of products that could be damaged by dust particles. ” Compare such a cleanroom with an operating room: there is a constant discharge of used air and a supply of fresh air, strict clothing regulations apply, there is a strict cleaning schedule and the working methods are constantly analyzed and monitored. OptimaT produces medical devices there that are sold in pharmacies.
Meet international requirements
Quality and constant improvement: OptimaT clearly pays a lot of attention to this. It also previously achieved the more widely known ISO9001 and ISO14001 standards. In order to also valorise the new efforts, the company decided to go for an ISO 13485 certificate. “We felt that this would add value for potential new customers,” says Marianne Deseure, financial and personnel director. "It's a way of demonstrating our ability to produce medical devices that consistently meet international safety, customer and regulatory requirements."
A rigorous audit process took place and was completed in September. "Our entire team has worked hard to make this happen. It shows that we are very committed to supplying quality products to a global market," says Quality Manager Lieselotte Vandenberghe. Marianne Deseure adds: “We are already well versed with quality management systems, but ISO 13485 is an extremely strict standard that is difficult to achieve. Logical, because it concerns medical devices. One small mistake can have major consequences for a user, ”she says. Just to illustrate how exceptional ISO 13485 is: the accreditation agency that OptimaT called upon had to send an employee from abroad to perform the audit.
Completely unburdening
The recognition shows that the company can completely unburden its customers. “A company that works with us can focus on its core business: R&D, design and sales. The entire production process can be done in-house with us. The fact that we have our own metal, powder coating and packaging department is a great asset. And our customers can also benefit from the strong reputation of the ISO 13485 certificate. They show that they have opted for an extremely professional approach for the production of medical materials. ”
OptimaT also wants to distinguish itself with its great commitment and flexibility. “Our technical department tailors the production processes to the customer. We will continue to optimize them ”, says Hein Moerman. “The cleanroom can also be adapted or expanded. We can, for example, combine it with an ESD zone (electrostatic discharge). There, the chance of electronic discharge is reduced to a minimum and we avoid damage to electronic components. ”
Moerman concludes with a reference to the corona crisis. “In the first wave, it became painfully clear how dependent our country was for medical devices on foreign suppliers. Isn't it great that we produce them locally, according to the highest quality standards, too? ”He concludes.